What is Ultraviolet (UV) radiation and usage of UV?
Ultraviolet (UV) light is found occupying the portion of the electromagnetic spectrum between X-rays and visible light. The sun emits ultraviolet light; however, much of it is absorbed by the earth’s ozone layer. Just as visible light consists of different colors that become apparent in a rainbow, the UV radiation spectrum is divided into three regions called UVA, UVB and UVC. As sunlight passes through the atmosphere. All UV-C and most UV-B are absorbed by ozone, water vapor, oxygen and carbon dioxide. UV-A is not filtered as significantly by the atmosphere. They differ in their biological activity and the extent to which they can penetrate the skin
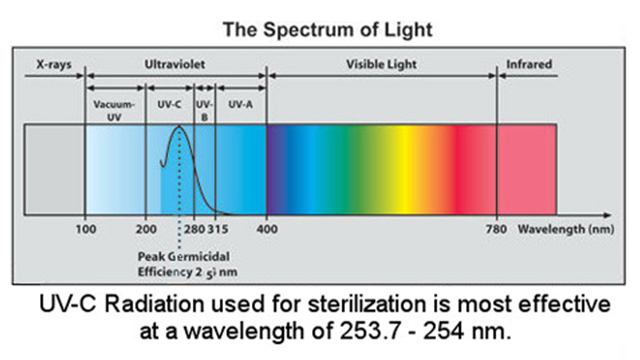
UV light or UV ray is classified into three components : Ultraviolet A (UVA), Ultraviolet B (UVB), and Ultraviolet C (UVC).
Following is the table explaining the characteristics of these components:
UV-A Wavelength: 315-400 nm. Not absorbed by the ozone layer
UV-B Wavelength: 280-310 nm. It is mostly absorbed by the ozone layer, but some does reach the Earth’s surface.
UV-C Wavelength: 100-280 nm. It is completely absorbed by the ozone layer and atmosphere.
Short-wavelength UV-C is the most damaging type of UV radiation.
A unique characteristic of UV light is that a specific range of its wavelengths, those between 200 and 300 nanometers (billionths of a meter), are categorized as germicidal – meaning they are capable of inactivating microorganisms, such as bacteria, viruses and protozoa. This capability has allowed widespread adoption of UV light as an environmentally friendly, chemical-free, and highly effective way to disinfect and safeguard water against harmful microorganisms.
Microorganisms are simple organic structures that readily absorb the UV-C wavelength, causing photo-disassociation (destruction). They are inactivated by UV light as a result of damage to nucleic acids. The high energy associated with short wavelength UV energy, primarily at 254 nm, is absorbed by cellular RNA and DNA. A microbes DNA (deoxyribonucleic acid) is the first to be adversely affected due to its weaker molecular bonds. In hundredths of a second it suffers irreparable damage. The subsequent loss of genetic instructions causes cell death and/or the inability to replicate, rendering them harmless. Continuous exposure causes uninterrupted degradation.
This UV-C light is also produced by electric arcs and specialized lights, such as mercury-vapor lamps.
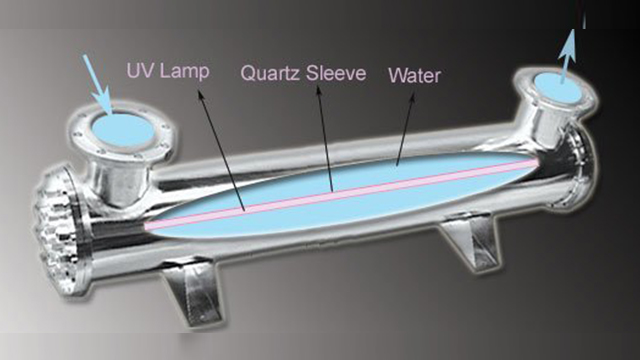
UV System : In this method water flows through the SS Chamber and gets in contact with submerged bank of germicidal lamps housed within quartz Sleeve.
Comparison of Ultraviolet v/s Chlorine & Ozone
| Methods | Ultraviolet | Chlorination | Ozone |
| Destruction | Physical | Chemical | Chemical |
| Capital cost | Low | Medium | High |
| Operating cost | Low | Medium | High |
| Maintenance cost | Low | Medium | High |
| Maintenance frequency | Low | Medium | High |
| Disinfection Performance | Excellent | Very Good | Unpredictable |
| Contact time | 1-5 Seconds | 25-45 minutes | 5-10 minutes |
| Personal Hazards | Low | Medium | High |
| Toxic Chemicals | No | Yes | Yes |
| Water Chemistry change | No | Yes | Yes |
| Residual effect | No | Yes | Yes |